- What is a vascular malformation?
- What is the typical natural history of a vascular malformation?
- Are vascular malformations cancerous?
- What are PTEN-associated vascular malformations?
- Are vascular malformations painful?
- Is a port wine stain birthmark a vascular malformation?
- What are some of the syndromes related to vascular malformations?
- How does an angiogram help diagnose vascular malformations?
- What are some treatments for vascular malformations?
- How does embolization or sclerotherapy treat a vascular malformation?
- Are there complications that can occur from treatment?
- What blood tests might be performed for patients with vascular malformations?
- Are vascular malformations inherited, and do genetics play a role?
- If my child has a vascular malformation, does that mean there may be others?
- Are vascular malformations affected by hormonal changes?
- Can there be vascular malformations in the brain?
- Can vascular anomalies be diagnosed prenatally (before birth)?
- What risks are associated with anesthesia, especially for children?
- When can blood clots form in a vascular malformation?
- Can women take birth control pills when they have a vascular malformation?
- Can a woman become pregnant and support a pregnancy when she has a vascular malformation?
- What are some medications used to treat patients with vascular malformations?
- What are the risks of not having a vascular malformation treated?
What is a vascular malformation?
Vascular malformations can affect veins, arteries, capillaries, and even lymphatic vessels. Vascular malformations are generally present prior to birth and grow in parallel with the overall growth of the patient. They are benign, or noncancerous, lesions. Even if they are present at birth, they may not become obvious until later, depending on the type and location of the malformation.
Vascular malformations affect males and females in equal frequency and can be located in any part of the body including the trunk, extremities, face, brain, or internal organs. They can affect one or several parts of the vascular system (e.g., they may affect the capillaries and veins, but not the arteries or lymphatic vessels) and are most precisely described based on the involved vessels. Clearly those affecting the skin are evident at birth.
Vascular anomalies are diagnosed through a variety of techniques. Sometimes vascular anomalies are obvious on the surface of the skin. With a thorough history and physical examination, a physician may be able to make the correct diagnosis by looking and touching the vascular anomaly. Sometimes, if the diagnosis is not obvious or typical, further testing is required, including blood tests, radiologic studies (e.g., ultrasound, x-rays, MRI). Sometimes a biopsy, which is a procedure to remove part of the lesion for diagnostic purposes, is required. In that case, a pathologist will look at the lesion under the microscope to examine the cells making up the vascular lesion and determine the diagnosis.
Figures provide images of patients with different vascular malformations.
What is the typical natural history of a vascular malformation?
The major difference between hemangiomas and vascular malformations is that the latter do not involute and are present throughout the patient’s lifetime. However, similar to hemangiomas, where they are located in the body and their clinical features determine how they are medically evaluated and managed.
For example, a capillary malformation (also called a port wine stain) presenting on the arm or abdomen may not cause concern, but one that covers the upper half or the entire face warrants evaluation for Sturge-Weber syndrome. Likewise, the presence of a large lymphatic malformation of the neck would require evaluation to monitor for airway compromise. High-flow vascular malformations such as arteriovenous malformations may present acutely with heart failure from arterial shunting and/or hydrocephalus due to blockage of cerebrospinal fluid drainage, as is the case with vein of Galen malformations in the brain.
The vast majority of vascular malformations are not associated with cancer.
Capillary malformation
Dilation of a cluster of small blood vessels (capillaries), which results in a visible reddish/ purplish coloring of the skin; also called a capillary malformation; it is present at birth.
Are vascular malformations cancerous?
While patients with rare vascular malformation syndromes (such as Maffucci syndrome or PTEN-associ-ated vascular malformations) may be predisposed to malignancies in adulthood, the vast majority of vascular malformations are not associated with cancer.
What are PTEN-associated vascular malformations?
PTEN stands for phosphatase and tensin homolog protein, encoded by the PTEN gene. PTEN is a tumor suppressor gene. In the normal state, PTEN prevents rapid cell growth. When mutated, this function of PTEN behaves abnormally, making patients with the PTEN mutation susceptible to cancers. Some patients with vascular anomalies have the PTEN mutation, such as those with Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome, and some patients with proteus syndrome. Some patients with vascular malformations may have the PTEN hamartoma tumor syndrome (PHTS), which represents a spectrum of disorders.
These syndromes are suspected if there are lipomas (benign fatty tumors), thyroid disorders, characteristic skin lesions, macrocephaly (large head), penile lentigines (freckles on the penis), or other associated findings. The only way to confirm the syndromes is to have a blood sample sent for PTEN mutational analysis; although there are several possible mutations, only the most common mutations can be tested. If an individual tests positive for this mutation, other family members will be tested, and early screening for thyroid, breast, brain, gynecologic, and other cancers will be initiated for those with the PTEN mutation.
Cowden syndrome is a rare disorder characterized by multiple noncancerous (benign) growths called hamartomas and an increased risk of developing certain cancers. Vascular malformations may be lymphatic, venous, or other. The skin growths, which are usually small, usually appear in the 20s, may look like warts or skin tags, and can be found on the skin and the mucous membranes (mouth, cheeks, gums, nasal passages).
Malignancies seen in patients with Cowden syndrome are usually breast, thyroid, or endometrium (lining of the uterus). Benign tumors are also seen in patients with Cowden syndrome—thyroid nodules, breast masses, and a noncancerous brain tumor called Lhermitte-Duclos disease (LDD), also referred to as dysplastic gangliocytoma of the cerebellum). Patients with Cowden syndrome may have a large head and cognitive delays. The diagnosis is often made in adolescence or early adulthood.
Often the suspicion of Cowden syndrome begins with a family history of thyroid nodules, lipomas (benign fatty lumps), and/or cancers, as previously noted. If the family history and clinical spectrum (macrocephaly, hamartomas, skin tag-appearing lesions) are present, the patient and family should be referred to a genetics specialist for further discussion and blood testing for the presence of the PTEN mutation. Since not all the mutations are available for testing, more sophisticated tests may be required if the initial test is negative and the patient/family fulfills the criteria for the disorder. Upon the suspicion or diagnosis of Cowden syndrome, individuals should be placed in a cancer surveillance program to facilitate early detection and prompt referral for further evaluation and treatment.
Bannayan-Riley-Ruvalcaba syndrome is characterized by macrocephaly (enlarged head), noncancerous fatty masses (lipomas), vascular malformations, intestinal polyps, thyroid disorders, pectus excavatum (caved-in appearing breast bone), hyperextensible joints, proximal muscle abnormalities, and predisposition to breast and thyroid cancers. Male patients have freckles on the penis. Bannayan-Riley-Ruvalcaba syndrome is also associated with mutations of the PTEN gene, thus the same guidelines hold true for patients suspected of having this disorder, as well as their family members. Bannayan-Riley-Ruvalcaba syndrome is often diagnosed in childhood.
Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome are inherited in an autosomal dominant fashion; thus, having only one copy of this abnormal gene is sufficient to manifest the disorder. Genetic testing can determine if other family members are affected or if this is a spontaneous mutation occurring only in the patient.
PTEN
Phosphatase and tensin homolog protein, a human gene that regulates cell division by preventing cells from dividing too rapidly; also acts as a tumor suppression gene.PTEN hamartoma tumor syndrome (PHTS)
Cluster of clinical findings (see Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome) associated with PTEN mutation.Lentigines
FrecklesCowden syndrome
Disorder characterized by macrocephaly, hamartomas, skin tag-appearing lesions, thyroid nodules, lipomas (benign fatty lumps) and/or cancers, and vascular malformations.Bannayan-RILEY-RUVALCABA syndrome
Macrocephaly (enlarged head), noncancerous fatty masses (lipomas), vascular malformations, intestinal polyps, thyroid disorders, pectus excavatum (caved-in appearing breast bone), hyperextensible joints, proximal muscle abnormalities, and predisposition to breast and thyroid cancers. Male patients have freckles on the penis.Autosomal dominant
Inheritance of a gene on a non-sex chromosome (i.e. not the X or Y chromosome) from one parent resulting in disease.
Are vascular malformations painful?
Day-to-day, vascular malformations are not usually painful. Patients may experience a sense of fullness, due to swelling, after walking or standing for prolonged periods. Use of compression stockings or wraps, or keeping the extremity elevated may decrease the discomfort. Phleboliths are calcifications of the remains of localized clots and only appear in a delayed fashion after the acute event. They are sometimes painful and can be relieved with oral ibuprofen-containing medications such as Advil or Motrin.
Development of a deep venous thrombosis is often associated with calf pain (or pain elsewhere depending on the location of the affected vessel). A Doppler study can document the blood clot. Anticoagulation therapy must be started promptly to prevent further clotting and possible dislodgement of the clot, which would travel to the lungs (causing a pulmonary embolism). Pain can also occur if a vascular malformation is located adjacent to a nerve, causing irritation and painful sensations.
In the peripubertal years, patients often experience bouts of discomfort, presumably due to hormonal changes to which the endothelial cells of the vascular malformations are sensitive.
Compression stockings
Therapeutic device used to support the venous and lymphatic system of the leg.
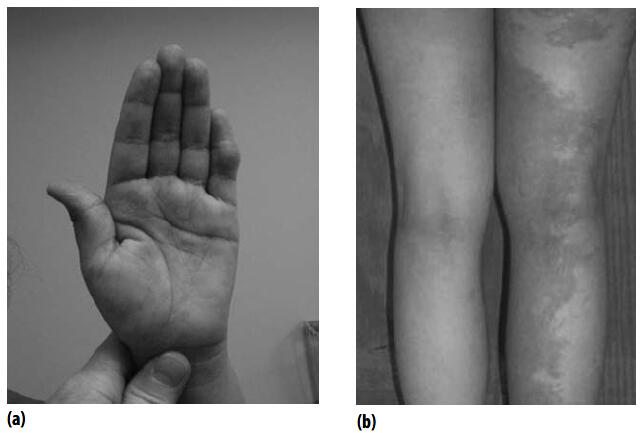
Is a port wine stain birthmark a vascular malformation?
Yes. A port wine stain is another term for capillary or venular malformations. These lesions are discolored areas on the surface of skin that range in color from pale pink to dark purple and are present at birth. They grow slowly at the rate of a child and do not spread to other areas. Over time they may become darker and thicker, especially if untreated.
Laser therapy is an effective treatment for port wine stains, but often a series of treatments is needed to produce a significant change. Lifelong maintenance treatments are usually required to maintain the lightened effect.
Depending on where port wine stains are located on the body, they may be a sign of Sturge-Weber syndrome or Klippel-Trenaunay syndrome, indicating that further neurologic and/or ophthalmologic evaluation is necessary.
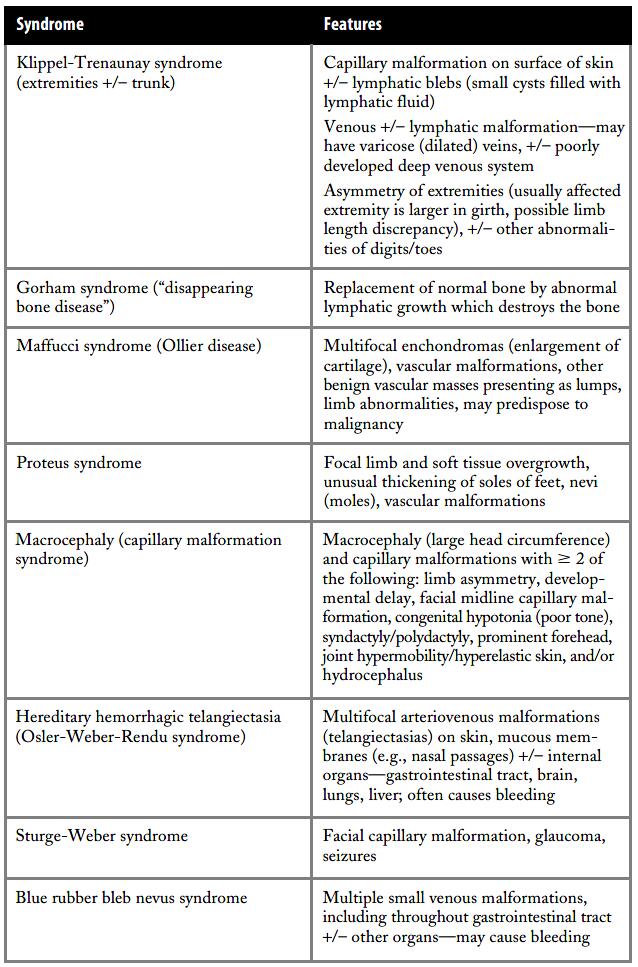
What are some of the syndromes related to vascular malformations?
In addition to the rare PTEN-associated vascular anomalyhamartoma syndrome, there are more common vascular anomaly syndromes as listed in the Table 4.
Sturge-Weber Syndrome
Sturge-Weber syndrome (SWS) is suspected when an infant is born with a facial capillary malformation. This malformation appears as a flat pink/red stain, and it can involve one or both sides of the face. Seizures, focal neurologic deficits (weakness, numbness, hemi-blindness) contralateral to the facial lesion, developmental delay, and glaucoma and may be associated with Sturge-Weber syndrome. Brain imaging has a typical pattern with areas of blood vessel proliferation (leptomeningeal angiomatosis), brain atrophy and calcification. SturgeWeber syndrome occurs equally in males and females. While a predisposing genetic mutation has not yet been identified, investigators are studying potential candidate genes which may be associated with this disorder. Some patients also have vascular staining on other parts of the body; however this is not a criterion for Sturge-Weber syndrome.
Another name for Sturge-Weber syndrome is encephalotrigeminal angiomatosis, as the full expression of the disorder includes the facial capillary malformation following the trigeminal nerve distribution, in addition to increased vascular growth in the leptomeningeal area of the brain. Seizures, glaucoma, and developmental delay, as well as various comorbidities such as attention deficit hyperactivity disorder (ADHD), depression, and hormonal abnormalities may be associated with Sturge-Weber syndrome. Glaucoma, the presence of increased intraocular pressure, if untreated can damage the optic nerve and cause blindness. Glaucoma can cause buphthalmos, enlargement of the affected corneas and eyeball.
Glaucoma may occur at any time and is often asymptomatic; therefore, patients must be monitored closely by an experienced ophthalmologist or glaucoma specialist, more frequently during infancy and early childhood, then minimally one to two times per year. Specifically, the ophthalmologist will check the intraocular pressure, which is elevated in glaucoma.
Researchers have found that MRI or MRA (magnetic resonance angiogram) brain imaging as well as a technique called quantitative EEG, or qEEG, can reveal important information about possible neurologic involvement. Neurologic symptoms may be acute (seizures) or subtle (onset of developmental delay). It is recommended that children at risk for Sturge-Weber syndrome be evaluated and followed regularly by an experienced neurologist who can discuss emergency measures in the event of a seizure and also the possible medical, educational, and social implications of this disorder. There are many different types of seizures. Medications used to treat seizures are called antiepileptics. Although general neurologists treat patients with seizures, neurologists who specialize in treating patients with seizures are called epileptologists. When medications are not able to control seizures, a patient may be evaluated for a vagal nerve stimulator implant or surgical resection of the seizure focus if indicated.
There are three categories of Sturge-Weber syndrome:
- Type 1: This type involves the face, brain, and eyes. These patients are prone to seizures, especially if both sides of the face are affected by the capillary malformation. The eye involvement may initially manifest as “red eye,” or extra capillaries on the surface of the sclera (white portion of the eyeball). Patients with all three anatomic areas of involvement are at highest risk for neurologic symptoms, including developmental delay.
- Type 2: This type involves a facial vascular staining with a normal brain and possible glaucoma.
- Type 3: In these patients, the forme fruste (incomplete, unusual) type of Sturge-Weber syndrome, there is brain involvement without cutaneous (skin) findings or glaucoma.
Not every child with a facial capillary malformation has Sturge-Weber syndrome. As noted, the risk increases when both sides of the face are involved. The National Institutes of Health and The Sturge-Weber Foundation provide helpful medical information and resources at their Web sites:
https://www.ninds.nih.gov/ (Search for “Sturge-Weber.”)
https://www.kennedykrieger.org/patient-care/centers-and-programs/sturge-weber-syndrome-center
The last Web site, for The Sturge-Weber Foundation (www.sturge-weber.org), has a host of resources for patients, including educational printed and audio-visual materials in English and multilingual translations, log books for medical visits and treatments, emergency room guides, online networking blogs, and suggestions for working with insurance companies.
Some researchers are conducting radiologic studies as well as testing if different medications and diet can prevent or modify symptoms such as seizures in patients with Sturge-Weber syndrome. There are other Sturge-Weber Foundation–funded researchers working on developing a mouse model and investigating immune suppression and hormone abnormalities. The Sturge-Weber Foundation Web site lists these centers as well as research projects they have recently funded.
Should your child be suspected of having SturgeWeber syndrome, he or she will be referred to an ophthalmologist for regular visits, a neurologist to follow neurodevelopment and to discuss the possibility of seizures and their management, and a laser specialist, who can help lighten or eradicate the capillary malformation with flashlamp pulsed dye laser treatments.
Management will then be tailored around the clinical findings and symptoms, if present. The SturgeWeber Foundation has designated “Sturge-Weber Centers of Excellence,” and these institutions have teams of doctors and support staff who have expertise in this disorder.
Sturge-Weber syndrome (SWS)
Medical condition with facial capillary malformation, often associated with seizures and glaucoma.Focal
Occurring in one location.Contralateral
Occurring on the opposite side.Leptomeningeal
Referring to the leptomeninges, one of the layers covering the brain.Trigeminal NERVe
Fifth cranial nerve with three major branches, mainly controlling feeling in the face (also controls some movements).Magnetic resonance angiogram
Special type of MR study focusing on the arterial structure.Flashlamp pulsed dye laser treatments
Laser treatment that improves red color of superficial vascular lesions; may prevent outward growth of early hemangiomas.
Klippel-Trenaunay Syndrome
Klippel-Trenaunay syndrome (KTS), a relatively common vascular anomaly syndrome, is named after the two French physicians who first described this disorder in 1900, Maurice Klippel and Paul Trénaunay. This syndrome describes a vascular malformation syndrome with anomalies of the vessels of an extremity (more commonly a lower extremity), hypertrophy of the affected extremity, and red or blue changes of the skin. These malformations may be associated with small blebs that ooze. Some patients with KlippelTrenaunay syndrome do not have a well-developed deep venous system. This is important to determine (usually by MRI), as the vessels close to the skin’s surface become dominant. Patients with Klippel-Trenaunay syndrome may also have lymphatic malformations or skeletal abnormalities such as extra digits, fused digits, or large feet or hands. Some patients also have anomalies of the abdominal, pelvic, and cranial vessels. Treatment is generally to relieve symptoms of pain and to prevent bleeding, clotting, and/or infections. Problems that can arise in these patients include limb length and girth discrepancies, blood clots, bleeding, cellulitis, lymphedema, and pain. However, day to day, many patients do very well. The medical team involved with patients having Klippel-Trenaunay syndrome often includes an orthopedist, interventional radiologist, dermatologist, hematologist, surgeon, and physiatrist. Helpful information can be found by searching for “Klippel-Trenaunay” on the Web sites of the following organizations:
- The Klippel-Trenaunay Syndrome Support Group
- National Institute of Neurological Disorders and Stroke
- Children’s Hospital Boston
- KT Foundation
- The Sturge-Weber Foundation
- MedlinePlus
Klippel-Trenaunay syndrome (KTS)
Vascular malformation syndrome associated with superficial vascular staining, hypertrophy of an extremity, and underlying venous and/or lymphatic malformation.Blebs
A blisterlike small cystic structure; may ooze or bleed.
Proteus Syndrome
Proteus syndrome (PS) or proteus-like syndrome is a congenital, asymmetric, disproportionate, and progressive postnatal overgrowth syndrome affecting bone, connective tissue, fat, and organs. Skin manifestations include characteristic cerebriform (brain-like configuration) changes, usually on the soles of the feet, and “linear epidermal nevus,” a brown, thick skin lesion. Hands and/or feet may exhibit gigantism (outsized growth). Patients with proteus syndrome have dysregulation of fatty tissue, with areas of fatty overgrowth alternating with fat atrophy; characteristic facial features; and capillary, venous, and/or lymphatic malformations. These individuals are at increased risk for malignancies. Proteus syndrome appears to occur sporadically and is considered a mosaic condition, which means individuals have a blend of affected (carrying a mutation) and unaffected cells. A multidisciplinary medical team, including a geneticist and orthopedist, is necessary for the proper monitoring and care of these patients.
Web sites with information about proteus syndrome include:
- Proteus Syndrome Foundation
- National Organization for Rare Disorders
Other Syndromes Related to Vascular Malformations
Macrocephaly-capillary malformation is an association of enlarged head (macrocephaly) with reticulated (network/weblike) capillary malformation, often with a midline facial vascular stain. Syndactyly (joined digits) and other abnormalities may be present.
Patients with blue rubber bleb nevus syndrome have multifocal venous malformations involving the skin, gastrointestinal (GI) tract, and soft tissues. Anemia often develops due to chronic bleeding from vascular malformations in the gastrointestinal tract. This symptom may manifest as dark or blood-coated stools, and patients may require blood transfusion to replace blood losses.
Patients with blue rubber bleb nevus syndrome have small protuberant soft dark blue discrete rubbery masses, evident on the skin and mucous membranes in areas such as the mouth and lips. Gastrointestinal lesions can be diagnosed by “capsule endoscopy” or “capsule enteroscopy” by which a tiny wireless camera inside a vitamin-sized capsule is swallowed by the patient or inserted by a gastroenterologist. Photographs of the intestinal tract are taken as the device travels throughout the digestive tract. The images are recorded on a monitor that enables the gastroenterologist to identify any abnormalities, including vascular malformations. Successful removal of problematic lesions by experienced surgeons has been described. Other vascular lesions in these patients may respond to sclerotherapy.
Maffucci syndrome is characterized by multifocal firm asymmetric subcutaneous enchondromas and dyschondroplasia; improper formation of bone in cartilage; and venous, lymphatic, or other vascular anomalies, including hemangioendotheliomas. The affected bones in patients with Maffucci syndrome have distinctive x-ray findings. They may suffer severe deforming orthopedic problems including limb length discrepancies, painful vascular nodules, and an increased incidence of cancers, including malignant transformation of enchondromas to chondrosarcomas, as well as cancers of the brain, breast, and genitourinary tract. Thus, these patients warrant early referral for orthopedic follow-up and close monitoring for early detection of cancer due to the 15–20% incidence of malignancies.
Macrocephaly
Enlarged head.Blue rubber bleb NEVUS syndrome
Condition with raised bluish nodules on the skin and throughout the gastrointestinal tract; may cause bleeding.Multifocal
Occurring in more than one location.Anemia
Low red blood cell count, leading to pallor.Maffucci syndrome
Disorder characterized by multifocal firm asymmetric subcutaneous enchondromas and dyschondropla- sia, improper formation of bone in cartilage, and venous, lymphatic, or other vascular anomalies.Enchondromas
Benign tumor of the cartilage.
How does an angiogram help diagnose vascular malformations?
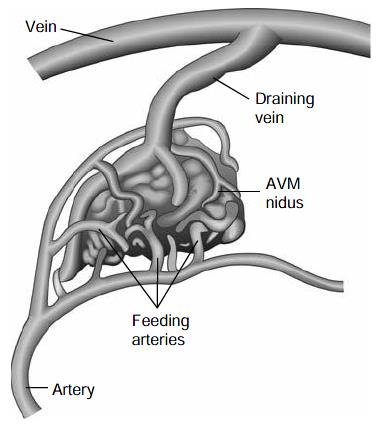
An angiogram is an imaging procedure that allows doctors to see blood vessels, including arteries and veins, and how blood moves within them (Figure).
This procedure is performed in a hospital setting by an interventional radiologist (a physician). During the procedure, the patient is sedated or put under anesthesia. The physician punctures the artery with a needle and a wire is threaded through the needle and into the vessels. Then a catheter (tube) is passed over the wire. A dye (contrast material) is injected into the vessel to allow the doctor to take x-rays of the vessels and their blood flow. This procedure can identify blockages, enlargements, and malformed vessels. Doctors may also be able to treat a problem during an angiogram, such as dissolving a clot by infusing a medication or performing an embolization to close off an unwanted vascular channel through the same catheter.
Angiogram is the general term for this test. If the test studies veins, it is called a venogram. If the arteries are studied, the test is called an arteriogram. After the procedure, the physician will review the images showing the malformations with you or your child.
Venogram
Diagnostic imaging test performed by an interventional radiologist. A catheter is inserted into the vessels of interest, and a contrast dye is injected to directly view blood flow of the venous system.
What are some treatments for vascular malformations?
Sometimes vascular malformations are removed surgically. This is often the case with small venous malformations or discrete lymphatic malformations. In other cases, embolization or sclerotherapy may be indicated; these therapies are utilized for arteriovenous, venous, and lymphatic malformations. Often management issues are discussed on a case-by-case basis among the involved physicians on the vascular anomalies team. In some cases, no treatment or intervention is required at all. New medical therapies may be available in a research setting.

In some cases, no treatment or intervention for vascular malformations is required at all.
How does embolization or sclerotherapy treat a vascular malformation?
Embolization and sclerotherapy are procedures that selectively close off blood vessels of a vascular malformation. Usually the treating physician, an interventional radiologist, will identify and map the vessels to be treated during an angiogram or venogram. If indicated, a substance will be injected using the same catheter to block the vessels in question or cause a local clot to shrink the lesion. Agents that are injected include ethanol, bleomycin, doxycycline, n-butyl-2-cyanoacrylate (n-BCA), ethylene-vinyl alcohol copolymer (Onyx), picibanil, and others. Sometimes sclerotherapy is performed via direct puncture into the lesion if it is superficially located and easily visualized. Embolization or sclerotherapy can be an effective treatment for vascular lesions in the brain or other parts of the body in the following conditions:
- Venous malformations
- Arteriovenous malformations
- Klippel-Trenaunay syndrome
- Kaposiform hemangioendothelioma
- Conditions in which bleeding needs to be minimized prior to surgical procedures
Embolization
Procedure by which a solution is injected into abnormal blood vessels or structures to create an obstruction or clot to close off the veins.
Sclerotherapy
Injection of a solution into abnormal blood vessels or structures to create an obstruction or clot to close off the vein.
Ethanol
Alcohol, sometimes used for endovascular treatment of vascular malformations.
Bleomycin
Antibiotic medication that may be injected into certain vascular malformations to make them shrink.
Doxycycline
Antibiotic sometimes used for sclerotherapy of vascular malformations.
Picibanil
Substance used for sclerotherapy of some lymphatic malformations.
Are there complications that can occur from treatment?
The risks and benefits of any intervention must be weighed. In the case of vascular malformations, the risks of treatment will depend on the affected location and extent of the lesion, age of the patient, any associated symptoms, and many other individual factors. These considerations will be discussed with you in detail by each of the involved physicians.
Some possible complications from surgery for vascular malformations are (1) the risk of malformation regrowth, (2) the loss or reduction of nerve function in an affected area, (3) the possibility of developing or mobilizing a clot, and (4) scarring. Compartment syndrome is a dangerous increase in pressure within a confined space, especially the calf or forearm. If untreated it can cause severe nerve or muscle damage and is a risk associated with surgery, embolization, and sclerotherapy, depending on the affected area. Procedures or diagnostic tests involving contrast dye may pose a risk to people with certain allergies, such as to iodine or shellfish.
Although these risks may sound frightening, many people with vascular anomalies who are treated by experienced specialists have very good outcomes and results with these, and other, procedures. If you have concerns or questions about specific risks associated with an intervention, it is best to discuss them with your physician(s).
What blood tests might be performed for patients with vascular malformations?
Because some patients with vascular malformations may have abnormalities in their blood counts, a CBC (complete blood count) may be performed. The white blood cells, which help fight infection, may be high if the patient has an infection. This may be the case in a patient who has a lymphatic malformation, which may be prone to inflammation and infection. Additionally, in patients with large lymphatic malformations, the lymphocyte count may be low.
The platelet count may be low in patients with venous malformations and localized intravascular coagulation (LIC) due to consumption of these blood products within the abnormal vessels. Other laboratory tests that may be abnormal are the coagulation profile (prothrombin time [PT] and partial thromboplastin time [PTT]), including the fibrinogen level (decreased) and the D-dimers and fibrin degradation products (FDPs), which may be elevated. These levels may alert the physician of a new development, and they may be followed serially to assess response to treatment.
For patients treated with anticoagulants, certain blood parameters are monitored. Patients treated with lowmolecular-weight heparin may be tested for antifactor Xa levels, and the PT/INR is followed in patients treated with Coumadin. Because of these potential blood test abnormalities, a hematologist may be involved in you or your child’s care. Figure table lists and describes these blood tests that help in the diagnosis and treatment of vascular malformations. Your physician will discuss these results with you.
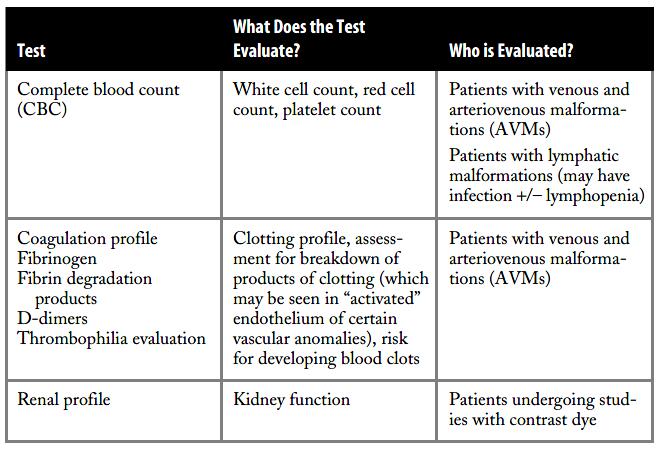
Figure depicts the blood vessel with the red blood cell and platelets (cellular components of the blood), in addition to factors involved with regulated clotting of blood.
The schematic of the coagulation cascade (Figure 11) depicts the main tests (activated PTT, PT, fibrinogen, fibrin degradation products) used for screening coagulation function in certain patients with vascular anomalies.
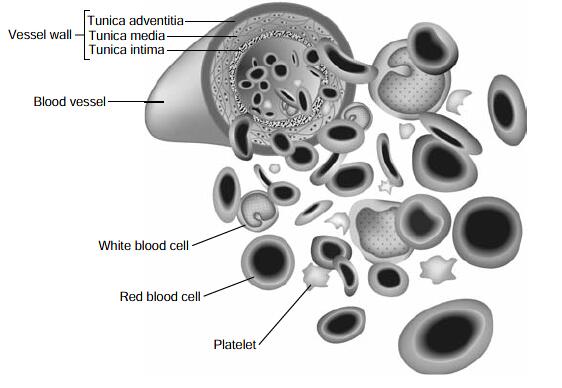
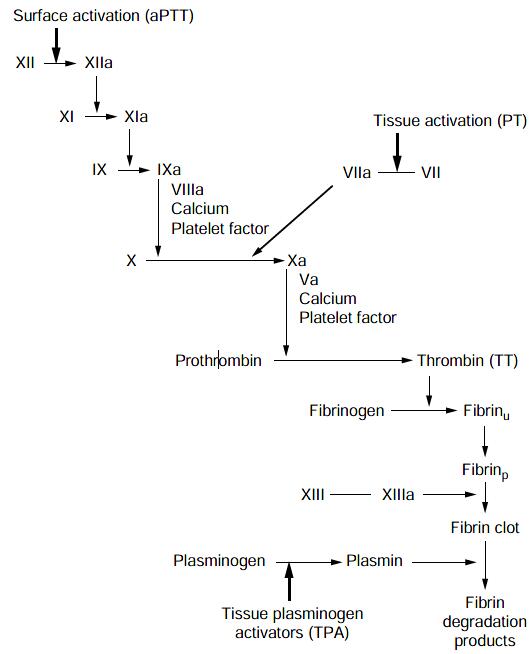
Anticoagulants
Medication or drug that prevents blood clotting.
Hematologist
Doctor specializing in treating diseases of the blood and clotting disorders.
Are vascular malformations inherited, and do genetics play a role?
Sometimes vascular malformations are inherited—that is, more than one generation is affected. A thorough family history may identify other family members who had similar types of vascular lesions. At this point, most vascular malformations do not have an identifiable genetic cause, although scientists are avidly investigating the potential genetic basis of these disorders and identifying new mutations every year.
Researchers have studied family patterns and identified certain genetic mutations associated with the affected individuals. Mutations of the following genes have been linked to familial vascular malformations: Tie-2, RASA-1, Glomulin, flt-4 (lymphatic), endoglin, ALK-1, and others. These genes play a role in various aspects of normal vascular development. If a patient is suspected of having a vascular anomaly for which a genetic mutation has been identified, blood samples from the patient and parents can be sent to laboratories studying these. Currently, the genetic testing for hereditary hemorrhagic telangiectasia (also known as Osler Weber Rendu), is the only approved (i.e., nonresearch) test for a genetic mutation associated with vascular anomalies (Figure). The initial testing is for mutations of endoglin or ALK-1. Once a mutation is identified, other family members can be easily screened for that mutation.
Your physician can discuss how genetic testing for other vascular malformations can be undertaken. A consultation with a genetics specialist and genetic counselor may also be recommended. Additionally, you or your child may be eligible for research projects in which blood samples are collected for genetic studies. Table 1 lists genetic mutations that have been identified in vascular malformations to date.
RASA-1
RAS p21 protein activator (GTPase activating protein) 1, CMAVM; CM-AVM
Hereditary hemorrhagic telangiectasia
Familial condition with multifocal arteriovenous malformations on mucosal surfaces (nasal passages, lips, gastrointestinal tract) and/or the brain, lungs, and liver.
If my child has a vascular malformation, does that mean there may be others?
Sometimes a vascular malformation extends beyond what is obvious. For this reason, the radiologic studies (often MRI) will include nearby structures. For example, a patient with a vascular malformation of the leg may have an MRI to study not only the leg but also the chest, abdomen, pelvis, and sometimes the brain.
Finding more extensive involvement is important as a baseline; however, in most cases, additional anomalies are not identified, and not all vascular malformations that are identified are symptomatic.
Are vascular malformations affected by hormonal changes?
Yes, vascular malformations may be responsive to hormonal changes. Some vascular anomalies are present at birth but often do not become apparent until years later, often during puberty. Hormonal changes related to puberty, pregnancy, and menopause can cause a vascular malformation to grow. In fact, even the hormonal changes related to a woman’s menstrual cycle can cause slight growth or changes to a vascular malformation. For example, a woman may notice slight swelling or mild discomfort in a vascular lesion around the time of her period.
Can there be vascular malformations in the brain?
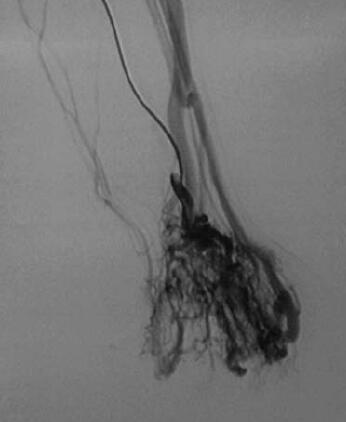
There are several vascular malformations that may affect the vessels of the brain. Some of these are diagnosed prenatally or shortly after birth, such as the vein of Galen malformation. Vein of Galen malformation is a type of arteriovenous malformation involving the vein of Galen. Emergency embolization may be necessary to control high-output cardiac failure if it does not respond to medical management. There is a risk of bleeding from any intracranial arteriovenous malformation, and if this occurs, there is a high mortality and morbidity (permanent deficits) rate; therefore, once the diagnosis is made, treatment is recommended if possible. Treatment is often endovascular therapy (i.e., angiogram with embolization, insertion of coils or other agents into the abnormal vessel) or surgery. Vascular malformations of the brain may first be diagnosed after a seizure, a hemorrhage, a stroke, localized neurologic deficits without stroke, an excruciating headache, vision disturbances, or memory lapses, or diagnosis may occur incidentally without symptoms. Immediate evaluation is necessary for diagnosis and appropriate treatment.
Patients with arteriovenous malformations (Figure 13) often experience a “swishing” noise or sensation.
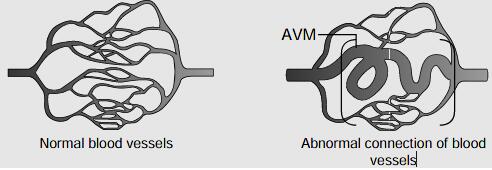
Dural fistulas occur when there is an abnormal connection between the blood vessels of the covering of the brain, the dura mater. A fistula is an aberrant, or abnormal, connection between an artery and a vein. There are several types of dural fistulas, depending on their location. Most common types are the following:
- Carotid cavernous sinus dural fistulas occur behind the eye, triggering ophthalmologic symptoms from augmented blood flow to the orbit. Patients have eye swelling, decreased vision, double vision, redness, and congestion of the eye.
- Transverse-sigmoid sinus dural fistulas occur behind the ear, causing headache, neck pain, and/or a blood flow sound in parallel with the heartbeat (a bruit). Seizure or hemorrhage can occur if there is blood flow from the malformation to the vein of the brain.
- Superior sagittal sinus dural fistulas occur toward the top of the head in the midline, leading to similar symptoms.
Helpful Web sites for further information include:
- American Stroke Association (Search for the term “AVM.”)
- Brain and Spine Foundation (See the online guide “Vascular Malformations of the Brain.”)
Many patients are curious to know if vascular anomalies of the brain are genetic. Patients with hereditary hemorrhagic telangiectasia may have intracerebral arteriovenous malformations. Cavernoma-type malformations of the brain vessels may also occur in families, in which case the lesions are often multifocal. In both of these disorders, mutations have been identified, which permits genetic screening of affected individuals and their families.
RASA-1 mutations have been identified in many patients with cranial arteriovenous malformations. Characteristically, these patients also have many localized flat red/pink capillary malformations on the body surface.
Vein of galen malformation
Structural malformation of an embryonic cerebral vein resulting in high flow arteriovenous shunting of blood—can result in neonatal high-output cardiac failure, stroke, hydrocephalus, and/or neurological deficits.
ENDOVASCULAR therapy
Treatment within the vessel via a catheter.
Can vascular anomalies be diagnosed prenatally (before birth)?
Sometimes vascular malformations are diagnosed prenatally by ultrasound and fetal MRI. Asymmetric limbs and/or high-flow lesions may be spotted this way. Also large cysts seen in lymphatic malformations may be detected prenatally.
Prenatal diagnosis may allow for medical/surgical in utero intervention before the baby is born. For example, a large hepatic hemangioma causing high blood flow may be treated in utero by having the mother take certain medications. High-flow RICH-type hemangiomas have also been managed by maternal ingestion of digoxin or other medications, which cross the placenta and in turn treat the fetus. Some large vascular anomalies (e.g., large lesions that may compromise the airway) may require careful preparation for delivery.* Rarely, fetal surgery is performed to avert a life-threatening situation.
Sometimes vascular malformations are diagnosed prenatally by ultrasound and fetal MRI.
*Sometimes the EXIT procedure (ex utero intrapartum treatment procedure) is necessary, whereby the infant is partially delivered via cesarean section, remaining attached by the umbilical cord until physicians establish a stable airway. This procedure requires a specially trained team of physicians, nurses, and ancillary med- ical staff. Identification of a large extremity or vascular mass may also indicate a cesarean section delivery.
What risks are associated with anesthesia, especially for children?
Modern anesthesia performed by experienced anesthesiologists is much less risky than in the past. Risks will vary depending on the extent of the procedure, the location and nature of the problem, and any associated conditions. Often the child’s pediatrician will be required to provide “medical clearance” in anticipation of anesthesia.
Additionally, other specialists (e.g., cardiologist, otolaryngologist, endocrinologist) will need to provide clearance as well. If the child has been on steroid treatment, stress dosing for the anesthesia may be required. It is best to discuss any questions with the involved physicians ahead of time.
Susan and Ken say:
Our doctor recommended that our daughter get an MRI. Because she was only 4 months old at the time, she was going to have to receive general anesthesia in order to do the MRI, and we were very worried about the risks associated with anesthesia in such a young child. We hesitated and stalled getting an MRI for a couple of weeks because we thought that having to “put our baby under” general anesthesia posed a greater risk than the growth of an internal hemangioma. At the suggestion of our doctor, we spoke in advance to the anesthesiologist, who was able to allay our fears. Although you can never totally eliminate anesthesia risk, in the hands of an experienced doctor who has administered anesthesia to many children, we felt that the risks were extremely low, and in the end, we were glad she got the MRI.
When can blood clots form in a vascular malformation?
Due to sluggish blood flow through abnormal veins, clots may form in vascular malformations. This will occur more frequently if a person is immobilized (e.g., bedridden), when legs are crossed (which stifles blood flow), and if there is an underlying hematologic predisposition to thrombose (clot). If there is a poorly developed deep venous system, then even blood clots in superficial veins may be clinically significant.
If a blood clot is small and in a superficial vein, it may feel like a small pea and feel tender for several days.
The pain often responds to Advil or Motrin. Deeper clots are treated with anticoagulation therapy. Generally, heparin or low-molecular-weight heparin (LMWH) is initiated. LMWH is administered via injection into the subcutaneous tissue twice daily. The dose is based on the patient’s weight. Some patients are transitioned to Coumadin, an oral medication. This drug requires more frequent blood testing. Newer oral anticoagulants that require less monitoring have been studied and will likely be on the market in the near future. See Question 24 for information about phleboliths.
Can women take birth control pills when they have a vascular malformation?
It is recommended that estrogen-containing birth control pills be avoided in women with vascular malformations, as unwanted blood clots can develop. Since birth control pills can be prescribed for a number of indications including irregular periods, acne, and hirsutism (extra unwanted hair growth), your doctor may discuss the potential side effects of birth control pills when your child is an early adolescent. Other forms of birth control are recommended.
Can a woman become pregnant and support a pregnancy when she has a vascular malformation?
In general, a woman who has a vascular malformation should be monitored closely during pregnancy and followed by a specialist in maternal fetal medicine, as many cases will be considered high-risk pregnancies and will warrant close monitoring of the mother and fetus.
Even women with large vascular malformations of the legs and pelvis have undergone successful pregnancies;
however, they benefit from being followed by an obstetrician familiar with patients who have complex medical issues. Women with vascular malformations may be treated with aspirin therapy or low-molecular-weight heparin (Lovenox) to prevent excessive blood clotting. Even with treatment, affected pregnant women may experience swelling of the vascular malformation, legs, vulva, and varicose veins of the legs.
LOVENOx
Agent administered subcutaneously to prevent further blood clots; brand name for Enoxaparin injection.
What are some medications used to treat patients with vascular malformations?
There is no medication that will “cure” a vascular malformation, although current management can result in clinical success and complete radiologic eradication of the malformations. Clinical trials are up and coming for those malformations that behave aggressively and cause complications such as skeletal deformities, bleeding disorders, pain, and organ damage.
Medications are generally prescribed on an as-needed basis to manage pain, infection, bleeding, or clotting. Procedures may also be recommended to improve or to prevent problems.
New research trials are in the pipeline, using medications to inhibit unwanted symptoms associated with vascular malformations, for the most challenging vascular malformations associated with progressive symptoms.
Susan and Ken say:
Our daughter was put on prednisolone at 4 months old to treat multiple facial hemangiomas. She was on prednisolone for 1 month, and after seeing very little progress, our doctor switched her to propranolol. Within only 24 hours, we saw substantial improvement, which has continued in the last few months. We feel very lucky that she reacted so well to the propranolol. There is no way to be sure how anyone will react to a particular medication, and you have to prepare yourself for the fact that it may take some time to find the right one for your child.
What are the risks of not having a vascular malformation treated?
Some vascular malformations do not require intervention and can be managed through observation. For other vascular malformations, treatment will help alleviate pain or discomfort, limb length and girth discrepancies, and psychosocial stigmata. With treatment, the lesions may be greatly improved, and if they are not cured they may be greatly improved functionally and cosmetically.
For patients with “aggressive” vascular malformations— a scenario that often arises in the peripubertal years—it may seem frustrating to only treat symptoms as they develop. Until we have improved medical therapies, however, symptomatic intervention is advised.